Are you a new member? Sign up now
Login area
| Sign up
Morbidity
| Chronic conditions | Prevalence |
|---|---|
| Cardiovascular diseases, atherosclerosis, coagulation problems and hypertension | 47% |
| Diabetes | 15% |
| Ulcers | 10% |
| High cholesterol level | 10% |
| Other diseases (Rheumatism, uric acid, glaucoma, osteoporosis...) | 8% |
| Diseases of the nervous system | 3% |
| Epilepsy | 3% |
| Thyroid Problems | 2% |
| Asthma | 2% |
*For previous years check the Statistical Bulletins' page
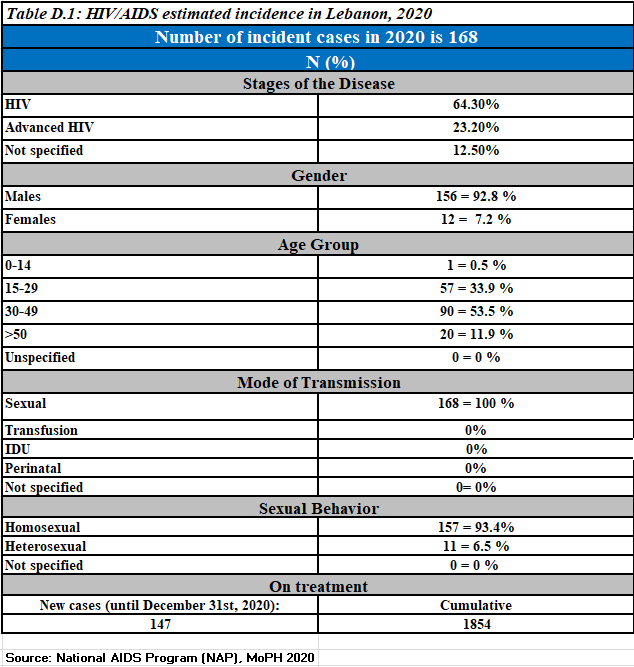
Newly reported HIV cases, 2020
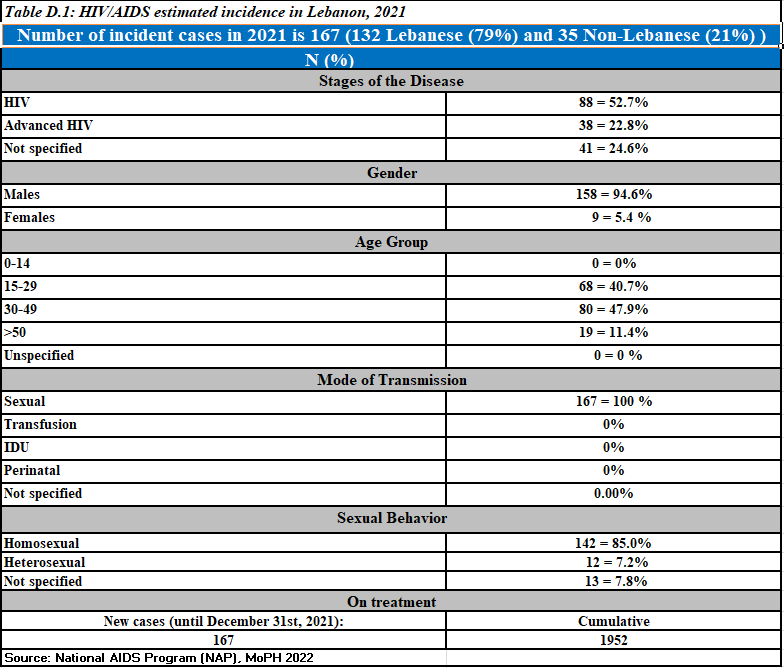
Newly reported HIV cases,2021
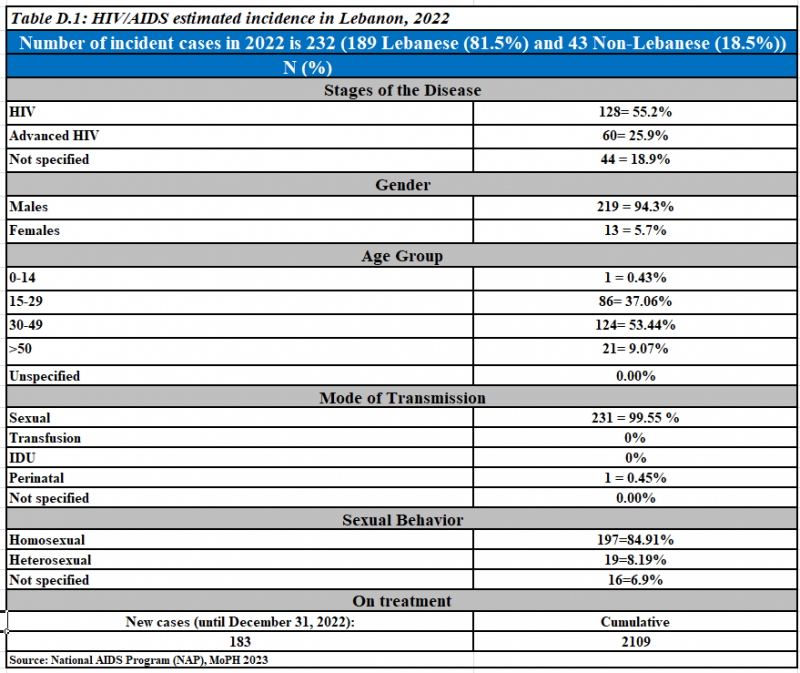
Newly reported HIV cases, 2022
Newly reported HIV cases, 2020

Newly reported HIV cases,2021

Newly reported HIV cases, 2022

Number of Measles cases
|
|
2012
|
2013
|
2014
|
2015
|
2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Measles
|
9
|
1,760
|
235
|
39
|
44 | 126 | 952 | 1070 | - | 28 | 95 | 351 |
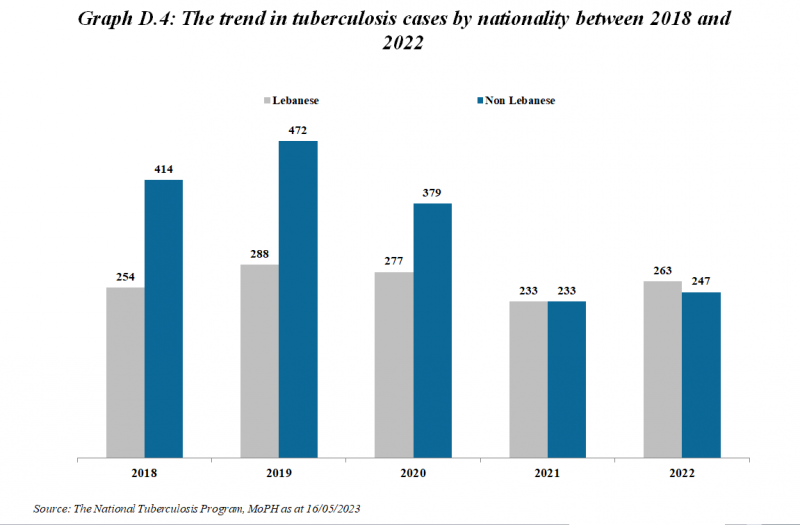
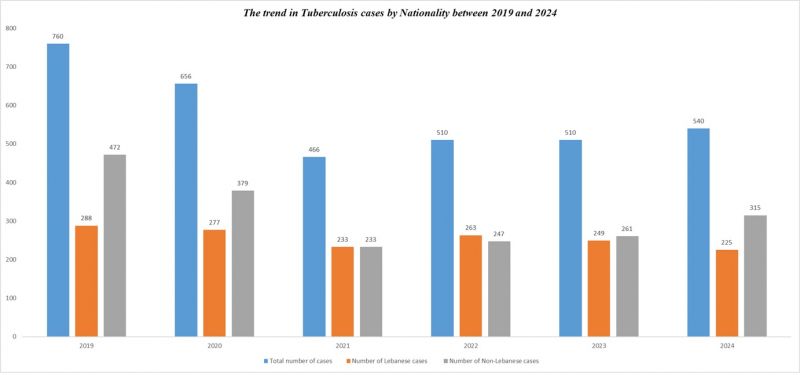
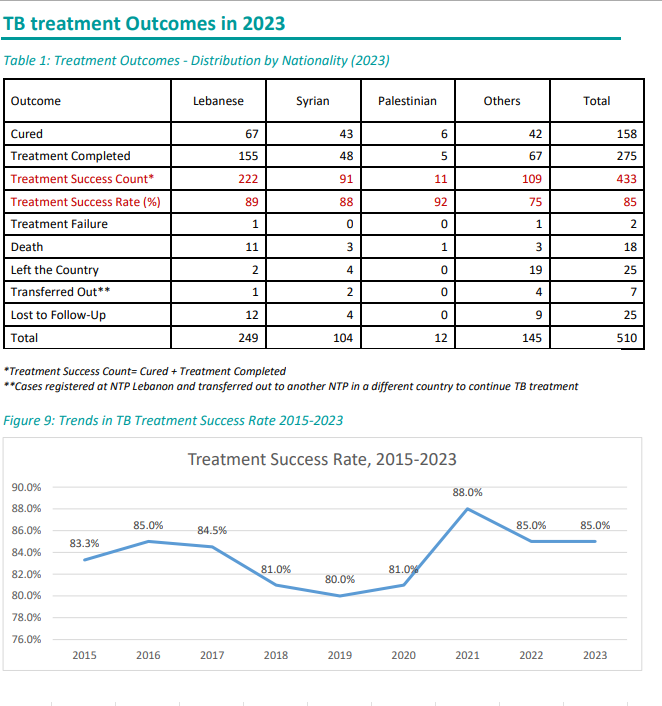
Number of Tuberculosis cases*
| Tuberculosis cases | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of cases | 630 | 689 | 682 | 666 | 657 | 632 | 668 | 760 | 656 | 466 | 510 | 510 | 540 |
| Number of Lebanese cases | 330 | 341 | 337 | 312 | 305 | 271 | 254 | 288 | 277 | 233 | 263 | 249 | 225 |
| Number of Non-Lebanese cases | 300 | 348 | 345 | 354 | 352 | 361 | 414 | 472 | 379 | 233 | 247 | 261 | 315 |
*MOPH National Tuberculosis Program 01/05/2025
The trend in Tuberculosis cases by Nationality between 2019 and 2024
The trend in Tuberculosis cases by Nationality between 2019 and 2024


Sitemap 

© Copyrights reserved to Ministry of Public Health 2025






.PNG)