Are you a new member? Sign up now
Login area
| Sign up
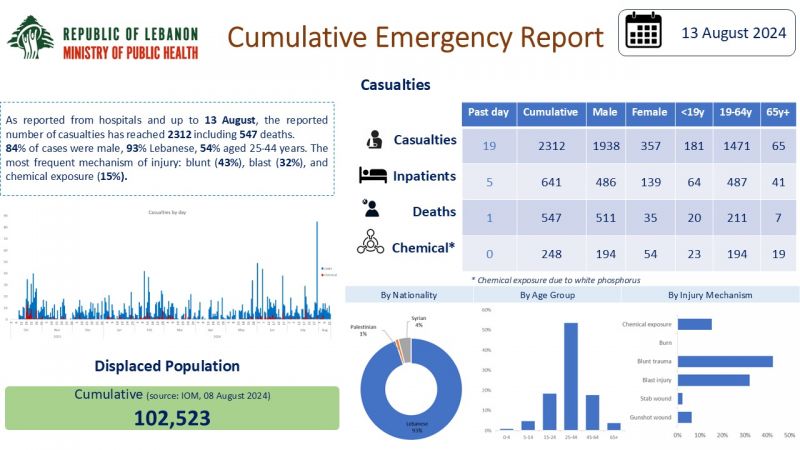
Cumulative Emergency Report-13-8-2024
In follow-up to the Israeli aggression against southern Lebanon, the Ministry of Public Health publishes the cumulative health emergency report for August 13, 2024.
For more information on the previous Cumulative Emergency Report, click on the following link: https://rb.gy/whsq0x
Sitemap 

© Copyrights reserved to Ministry of Public Health 2025