Are you a new member? Sign up now
Login area
| Sign up
MediTrack Project - Track & Trace System for Pharmaceuticals
Project Definition
Strategic Framework- This project comes within the plan to reform and develop the pharmaceutical sector in Lebanon by reviewing legislation, regulating the process of drug registration and pricing, and formulating quality assurance standards and programs with the aim of providing safe and quality-assured medicine at the right price for the patient.
- The E-Health Program team at the Ministry of Public Health implements and manages this project under the leadership of the Program Director, Mrs. Lina Abu Mrad, and in coordination with the Pharmacy Department and its affiliated departments and the IT Department, and in cooperation with all concerned authorities and stakeholders in the health sector.
- This project is being implemented with the support and funding of the World Health Organization, the Italian government, and the European Union, where the necessary hardware and equipment are provided, including servers, computers and barcode readers.
Project Goals and Importance
- Ensuring safe drug access to the patient.
- Controlling the dispensing of subsidized medicines.
- Preventing medicines storage.
- Visibility and Protection from the point of manufacture to sale to the consumer.
- Production process compliant with regulations.
- Reducing counterfeited and illegal drugs.
- Fast recalls from the market.
- Fast Reimbursement and fraud control at the third party payers
Project Summary
- Work has begun on this project in the Ministry of Public Health since 2017, several regulatory decisions have been issued with the aim of implementing the 2D Matrix Barcode on all medicines registered or intended to be registered at the Ministry of Public Health by all pharmaceutical institutions and pharmaceutical factories duly registered in the Ministry of Public Health and which intends to manufacture or import medicines.
- Barcode Structure
| AI Description | Numbers |
|---|---|
| GTIN | 1 |
| Batch or Lot # | 10 |
| Expiry Date | 17 |
- In 2017, the Ministry of Public Health developed an information system to track and trace the medicines (MediTrack) within the supply chain from manufacturing, to storage, distribution and dispensing to patients through a unified drug identification number (2D Matrix barcode).
- The pilot phase of MediTrack began in March 2018, in which about 19 pharmaceutical institutions participated, till the end of 2018, as per the ministerial decision No. 2428 dated 12/14/2017.
- In 2020, the Lebanese Ministry of Public Health issues guidelines, according to the international guidelines of the GS1, and stresses the importance of adhering to this manual by all parties involved, as relevant to the particular role that they play, believing that the barcoding technology adds an extra level of patient safety to the medication administration process.
- At the end of 2020, the MediTrack system was implemented with drug warehouse owners in Lebanon, and all importers and distributors of medicines in Lebanon were connected to the information system to track the movement of imported medicines.
- In the summer of 2022, the decrease in the budget allocated by the Central Bank of Lebanon for total subsidies for catastrophic illnesses led to an urgent need to track the dispensing of subsidized medicines up to the patient level in both the public and private sectors. The Ministry of Public Health took this opportunity to accelerate the implementation of the MediTrack system in hospitals and pharmacies as a way to control the sale of subsidized cancer medicines and to prevent the storage of medicines or their transfer to foreign markets.
- Since August 2022, pharmaceutical institutions (hospitals, pharmacies, pharmaceutical factories, importers, distributors, security forces, donors, non-governmental organizations and health care centers...) have been trained and connected to the MediTrack system because the dispensing of some cancer drugs and other subsidized drugs must be done exclusively on the MediTrack system as per Minister Decision No. 6/1 issued on 5/1/2023. The patient must be registered in a center identified by MoPH to have a Unique Health ID as a condition for obtaining the treatment.
- Since the beginning of this year 2023, the national pharmaceutical manufacturers have also started working on applying and printing two-dimensional barcodes on all locally manufactured medicines. They have also been trained on MediTrack system and they started working on it. Thus, all medicines in the Lebanese markets are now tracked within the supply chain.
- From 2017, several decisions and regulatory documents (more than 30) have been issued to keep pace with the implementation and development of this project.
- Coordination is made on a permanent basis with the Department of Pharmacy in legislative matters, especially with regard to pharmaceutical institutions and the organization of the drugs registration and pricing process. Coordination is also made with the Pharmacy Inspection Department in terms of checking and validating the information of imported drugs and their invoices entered on MediTrack by importers in order to to approve their distribution.
- Cooperation is also taking place with the IT Department regarding the implementation of the “Unique Health ID” platform and training of registration centers identified by MoPH to use this platform to register patients and provide them with their Unique ID in order to have access to treatment.
Information Flow
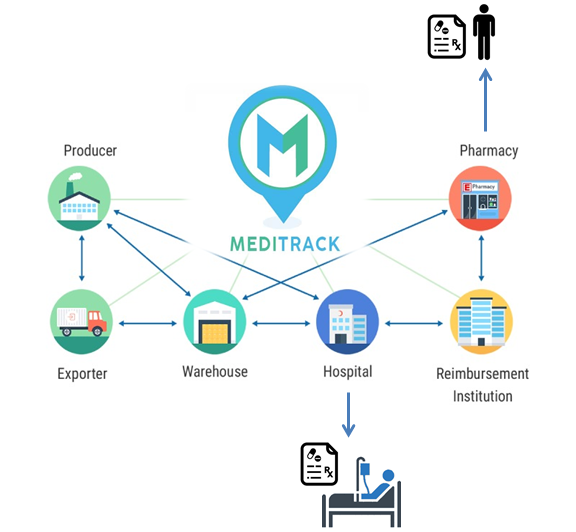
Administrative Procedures for Patient's Access to Drugs through Meditrack
للحصول على الدواء
مريض السرطان:
- الحصول على رقم صحي موحد
- التواصل مع طبيبك المختص لتعبئة ملف خاص بك على منصة امان
- انتظار رسالة نصية من وزارة الصحه تبلغ المريض اذا كان ( مقبول، مرفوض، قيد الدرس ) بحالة القبول عليه المتابعه مع المستشفى اذا كان الدواء يعطى من خلال الوريد، في حال كان الدواء يعطى على شكل حبوب / حقن سيتم التواصل مع المريض من خلال فريق عمل وزارة الصحه خلال مهلة اسبوع لمعرفة الصيدليه القريبه من السكن و ارسال الدواء اليه من ثم يتابع المريض بعدها مع الصيدليه شهريا .
مريض التصلب اللويحي :
- الحصول على رقم صحي موحد
- التواصل مع طبيبك المختص لتعبئة ملف خاص بك على منصة امان
- انتظار رسالة نصية من وزارة الصحه تبلغ المريض اذا كان ( مقبول، مرفوض، قيد الدرس ) بحالة القبول عليه المتابعه مع المستشفى اذا كان الدواء يعطى من خلال الوريد، في حال كان الدواء يعطى على شكل حبوب / حقن سيتم التواصل مع المريض من خلال فريق عمل وزارة الصحه خلال مهلة اسبوع لمعرفة الصيدليه القريبه من السكن و ارسال الدواء اليه من ثم يتابع المريض بعدها مع الصيدليه شهريا.
مريض الهيمو فيليا :
- الحصول على رقم صحي موحد
- التواصل مع طبيبك المختص لتعبئة ملفك الصحي على منصة امان
- أو تقديم المستندات التالية إلى وزارة الصحة العامة او اقرب مركز لها أو ارسالها عبر رسالة واتساب على الرقم التالي 81/348838
المستندات المطلوبه هي :
صورة عن الهوية، رقم صحي موحد، تقرير طبي مفصل (مع ذكر الدواء والعيار والجرعة اليومية)، وصفة طبية موحدة.
تقوم الشركة بتسليم الدواء للصيدلية المحددة او عبر الكرنتينا اذا كان المريض غير مضمون او عبر مراكز الدواء الموزعه بالمراكز.
تستغرق هذه العملية تقريبا ٥ ايام عمل بدء من وقت تسليم المستندات المتعلقة بالمريض لجانب وزارة الصحة العامة
مرضى التلاسيميا :
صورة عن الهوية، رقم صحي موحد، تقرير طبي مفصل (مع ذكر الدواء والعيار والجرعة اليومية)، وصفة طبية موحدة.
تقوم الشركة بتسليم الدواء للصيدلية المحددة او عبر الكرنتينا اذا كان المريض غير مضمون او عبر مراكز الدواء الموزعه بالمراكز.
تستغرق هذه العملية تقريبا ٥ ايام عمل بدء من وقت تسليم المستندات المتعلقة بالمريض لجانب وزارة الصحة العامة
مرضى التلاسيميا :
- الحصول على رقم صحي موحد
- التواصل مع طبيبك المختص لتعبئة ملفك الصحي على منصة امان
- أو تقديم المستندات التالية إلى وزارة الصحة العامة او اقرب مركز لها أو ارسالها عبر رسالة واتساب على الرقم التالي 81/348838
المستندات المطلوبه هي :
صورة عن الهوية، رقم صحي موحد، تقرير طبي مفصل (مع ذكر الدواء والعيار والجرعة اليومية)، وصفة طبية موحدة.
مرضى الزرع: دواء Advagraf or Prograf
صورة عن الهوية، رقم صحي موحد، تقرير طبي مفصل (مع ذكر الدواء والعيار والجرعة اليومية)، وصفة طبية موحدة.
مرضى الزرع: دواء Advagraf or Prograf
- الحصول على رقم صحي موحد
- التواصل مع طبيبك المختص لتعبئة ملفك الصحي على منصة امان
- او اعطاء وزارة الصحه المستندات التاليه :
- تقرير طبي جديد (تقرير طبي جديد يقدم لمرة واحدة يبين ان المريض اجريت له عملية زراعة عضو وهو بحاجة لهذا الدواء مدى الحياة) .
- رقم صحي موحدد
- وصفة طبية جديدة لا تقل عن شهر ( مع ذكر الدواء والعيار والجرعة اليومية) وارسالها عبر رسالة واتساب على الرقم التالي 81/348838.
تقوم الشركة بتسليم الدواء للصيدلية المحددة او عبر الكرنتينا اذا كان المريض غير مضمون او عبر مراكز الدواء الموزعه بالمراكز
تستغرق هذه العملية تقريبا ٥ ايام عمل بدء من وقت تسليم المستندات المتعلقة بالمريض لجانب وزارة الصحة العامة
دواء من الهبة المقدمة بشكل استثنائي لفترة ثلاثة اشهر : Humira
الحصول على رقم صحي موحد
التوجه لوزارة الصحه الطابق الرابع لاعطائها المستنداتت التاليه :
تستغرق هذه العملية تقريبا ٥ ايام عمل بدء من وقت تسليم المستندات المتعلقة بالمريض لجانب وزارة الصحة العامة
دواء من الهبة المقدمة بشكل استثنائي لفترة ثلاثة اشهر : Humira
الحصول على رقم صحي موحد
التوجه لوزارة الصحه الطابق الرابع لاعطائها المستنداتت التاليه :
- رقم الصحي الموحد
- صورة عن الهويه
- تقرير طبي مفصل من الطبيب المختص (روماتيزم، جهاز هضمي، العيون و الجلديه )
- وصفه طبيه موحدة ( مع ذكر اسم الدواء والجرعه المسموح فيها شهريا )
- فحوصات مخبريه : sgot, sgpt, creatinine, cbc
Pharmaceutical Companies Connected to Meditrack System
- List of Public Hospitals Certified For the Unique Health ID Registration updated on 22/09/2025
- List of Private Hospitals Certified For the Unique Health ID Registration updated on 22/09/2025
- List of Hospitals Connected to "Meditrack System" updated on 12/1/2026
- List of Pharmacies ِApproved by the Ministry of Public Health to Distribute Free Medications for Incurable Diseases through "Meditrack System" updated on 12/1/2026
- List of Pharmacies Connected to "Meditrack System" updated on 12/1/2026
List of Medications Dispensed through the Meditrack System
- List of Medicines for Incurable Diseases Distributed Free of Charge by the Ministry of Public Health updated on 29/1/2026
- List of Medications within the E1 and E2 Categories that are Dispensed through the Meditrack System, Based on Ministerial Decision No. 264 issued on February 3, 2025
Guidelines for Pharmacy Stock Management Systems Integration with MediTrack System
- API documentation that is needed by the pharmacy software companies to start the integration with MediTrack are listed below:
- The test environment base URLs:
Nevertheless, please be aware that we have two API services at MOPH, namely
/api and /meditrackapi.
You can find these services at the following URLs:
1. https://meditrack.moph.gov.lb:38440/api
2. https://meditrack.moph.gov.lb:38440/meditrackapi
It is essential to obtain the token from the same application (same BaseURL)
Patient UHID base URL: https://meditrack.moph.gov.lb:38440/meditrackapi/
Pharmacy Movements base URL: https://meditrack.moph.gov.lb:38440/api/
Pharmacy Sales base URL: https://meditrack.moph.gov.lb:38440/
Guidelines-Implementation of the 2D BarCode on Pharmaceuticals
In order to ensure the patient’s access to safe drugs, the Ministry of Public Health has adopted the two-dimensional (2D) barcode as a unified identification number on the package of the pharmaceutical products. The 2D barcode will help trace these products within the supply chain, from manufacturing, to storage, distribution and receipt of drugs by patients.
It has therefore become necessary to develop this manual designed to ensure the adoption of the necessary standards to include the barcode on drugs and help seize smuggled and counterfeit drugs in the Lebanese market.
It has therefore become necessary to develop this manual designed to ensure the adoption of the necessary standards to include the barcode on drugs and help seize smuggled and counterfeit drugs in the Lebanese market.
Guidelines-Implementation of the 2D BarCode on Pharmaceuticals-Edition2-2020 (update related to the barcode reader configuration)
Director General Letter 2020/4/15753 in 22/5/2020
Laws And Regulations
- Memo No.133/1 of 24/12/2019 on the exemption of some pharmaceutical products from barcode implementation- Attached List of exemption of some pharmaceutical products from barcode implementation - Updated on 21/1/2026
- Memo No. 43 issued on 14/4/2025
- Minister's Decision No.264 Issued on 3/2/2025 Regarding the Work of Pharmaceutical Institutions on MediTrack
- Memo No. 33 issued on 31/10/2024 regarding Clarification on medications purchased under Public Tender No. 388/2024.
- Minister's Decision No. 988/1 issued on 21/8/2023 concerning the implementation of relevant ministerial decisions related to MEDITRACK
- Minister's Decision No. 412/1 of 25/4/2023 on Linking the Pharmacy Inventory Management System POS to MediTrack
- Minister's Decision No. 276/1 of 14/3/2023 on the Amendment of Decision No. 228/1 of 6/3/2023 (Dispensing Subsidized Drugs for Cancer and Incurable Diseases at Hospitals and Pharmacies)
- Minister's Decision No 230/1 of 8/3/2023 on the Amendment of Decision No 945/1 of 3/10/2022 (Mechanism for Dispensing Drugs)
- Minister's Decision No.229/1 of 6/3/2023 on the Amendment of Decision no. 6/1 of 5/1/2023 at Health and Pharmaceutical Institutions For Tracking Cancer and Incurable Disease Drugs
- Minister's Decision no 228/1 of 6/3/2023 on the Amendment of Decisions no. 7/1 and 8/1 of 5/1/2023 (Dispensing Subsidized Drugs for Cancer and Incurable Diseases at Hospitals and Pharmacies)
- Minister’s Decision No. 8/1 of 05/01/2023 on Dispensing Subsidized Drugs on MediTrack System at Pharmacies
- Minister’s Decision No. 7/1 of 05/01/2023 on Dispensing Subsidized Drugs at Hospitals
- Minister’s Decision No. 6/1 of 05/01/2023 on the Introduction of the Trial Period for the Unique Health ID – MediTrack Systems At Health and Pharmaceutical Institutions For Tracking Cancer Drugs
- Minister's Decision No. 1192/1 of 29/11/2022 on inspection of Importers' invoices on MediTrack
- Minister's Decision No. 945/1 of 3/10/2022 on the Establishment of a Mechanism for Dispensing Medicines
- Minister's Decision No.647/1 of 13/7/2022 on the Preparations Needed for the Implementation of the Barcode on the Locally Manufactured Medicines
- Minister's decision No. 1083/1 of 10/9/2021 on the formation of a technical commitee to follow up the implementation of Meditrack system and the medicine card project
- Circular No 65/1 of 10/9/2021 on facilitating the work on Meditrack platform
- Memo No. 30/ of 24/3/2021
- Circular No 29/1 of 24/3/2021
- Minister's decision No. 1476/1 of 3/11/2020 on proceeding with the implementation of MediTrack computer system by drugs importers and warehouse owners
- Minister's Decision No. 1242/1 of 18/9/2020 on the implementation guidelines of the 2D Matrix Barcode for pharmaceutical products
- Minister's Decision No.1253/1 of 18/9/2020 on the adoption of the 2D Matrix barcode for vaccines
- Memo No. 107/1 of 17/7/2020 on the amendment of memo No. 132 of 24/12/2019
- Memo No.16/1 of 7/2/2020 on the request of information from parmaceutical companies
- Minister Decision No. 2648/1 of 24/12/2019 - Annexe 1: Barcode Information for Imported Drugs
- Memo No.132/1 of 24/12/2019 on the extension of the deadline for the implementation of barcode on some pharmaceutical products
- Minister's Decision No. 2291/1 of 7/11/2019 on printing the expiry date on drug packages
- Minister's Decision No. 1894/1 of 17/9/2019 on the necessary preparations for the implementation of barcode on drugs
- Minister's Decision No. 1893/1 of 17/9/2019 : updating drug barcode application forms
- Minister's Decision No. 2062/1 of 19/10/2018 on the extension of the deadline for the implemetation of barcode on drugs
- Minister's Decision No. 1424/1 of 27/7/2018 on the compliance with the 2D Mtrix barcode on drugs
- Minister's Decision No. 2429/1 of 14/12/2017 on sharing the GTIN number of drugs
- Minister's Decision No. 2428/1 of 14/12/2017 on the time line for the implemtation of the 2D Matrix barcode on drugs
- Minister's Decision No. 2405/1 of 11/12/2017 on the adoption of the 2D Matrix barcode
- Minister's Decision No.1817/1 of 2/10/2017 on the amendment of decision No.1432/1 related to the formation of a committee for the examination of the implementation of barcode and the unified medical prescription
Presentations
- Tracking cancer drugs through MediTrack - Training session for public and private hospitals- 26/11/2021
- MediTrack Integration with MVC- 2/9/2021
- 2D Barcode Project Updates- 25/11/2021
- Presentation of Mrs. Lina Abou Mrad- 20/1/2020
- Presentation of Dr. Walid Ammar- 11/12/2017
Contacts Us
For any support on MediTrack, you can reach us during the working hours at MoPH as follows:By Email: meditrack@moph.gov.lb
By Phone:
01-830292 - Hussein El Masri
01-830263 - Tony Nasrallah
01-830268 - Mariane Bazzi
For any support on Unique Health Id system, please contact Mr.Hicham Awad
By Email: awad.hicham@gmail.com
By Phone: 03-659442
Media
Related video

Related video

Sitemap 

© Copyrights reserved to Ministry of Public Health 2026
























